



2.900 Å
X-ray
2006-05-02
| Name: | Adenylate cyclase type 2 | Adenylate cyclase type 5 |
|---|---|---|
| ID: | ADCY2_RAT | ADCY5_CANLF |
| AC: | P26769 | P30803 |
| Organism: | Rattus norvegicus | Canis lupus familiaris |
| Reign: | Eukaryota | |
| TaxID: | 10116 | 9615 |
| EC Number: | / | |
| Chain Name: | Percentage of Residues within binding site |
|---|---|
| A | 61 % |
| B | 39 % |
| B-Factor: | 38.923 |
|---|---|
| Number of residues: | 31 |
| Including | |
| Standard Amino Acids: | 29 |
| Non Standard Amino Acids: | 2 |
| Water Molecules: | 0 |
| Cofactors: | |
| Metals: | MN |
| Ligandability | Volume (Å3) |
|---|---|
| 0.832 | 587.250 |
| % Hydrophobic | % Polar |
|---|---|
| 42.53 | 57.47 |
| According to VolSite | |
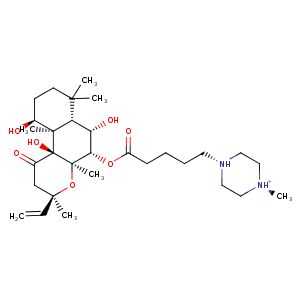
| HET Code: | FKP |
|---|---|
| Formula: | C30H51N2O7 |
| Molecular weight: | 551.735 g/mol |
| DrugBank ID: | - |
| Buried Surface Area: | 54.41 % |
| Polar Surface area: | 120.97 Å2 |
| Number of | |
|---|---|
| H-Bond Acceptors: | 8 |
| H-Bond Donors: | 4 |
| Rings: | 4 |
| Aromatic rings: | 0 |
| Anionic atoms: | 0 |
| Cationic atoms: | 1 |
| Rule of Five Violation: | 1 |
| Rotatable Bonds: | 8 |
| X | Y | Z |
|---|---|---|
| 45.1013 | -10.3023 | 50.2142 |
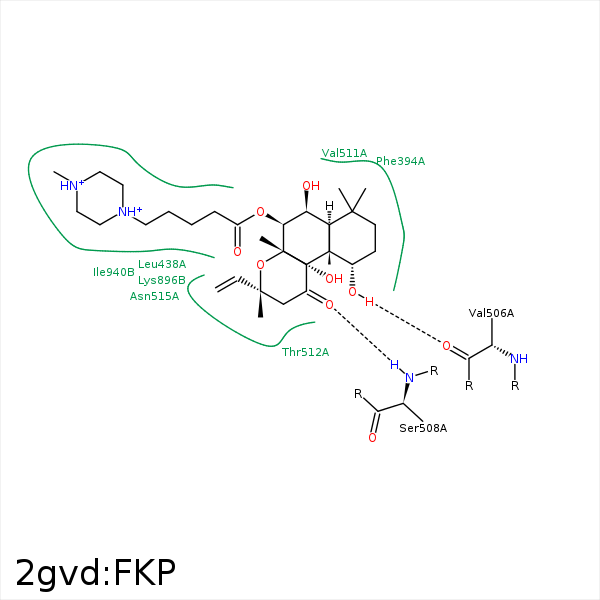
 Image generated by PoseView
Image generated by PoseView



Represent the protein/ligand binding mode, centered on the ligand
Dashed lines represents hydrogen bonds and metal interactions
Green residue labels for amino acids with hydrophobic contacts (green lines) to the ligand

| Ligand | Protein | Interaction | |||
|---|---|---|---|---|---|
| Atom | Atom | Residue | Distance (Å) | Angle (°) | Type |
| C2 | CZ | PHE- 394 | 3.7 | 0 | Hydrophobic |
| C3 | CE2 | PHE- 394 | 4.27 | 0 | Hydrophobic |
| C19 | CE2 | PHE- 394 | 4.09 | 0 | Hydrophobic |
| C18 | CD1 | LEU- 438 | 3.93 | 0 | Hydrophobic |
| C2 | CE2 | TYR- 443 | 4.3 | 0 | Hydrophobic |
| C3 | CZ | TYR- 443 | 4.04 | 0 | Hydrophobic |
| O2 | O | VAL- 506 | 2.69 | 159.95 | H-Bond (Ligand Donor) |
| O7 | N | SER- 508 | 3.27 | 169.72 | H-Bond (Protein Donor) |
| C2 | CG1 | VAL- 511 | 3.63 | 0 | Hydrophobic |
| C17 | CG2 | THR- 512 | 4.17 | 0 | Hydrophobic |
| C20 | CB | THR- 512 | 4.17 | 0 | Hydrophobic |
| C19 | CB | ASN- 515 | 4.24 | 0 | Hydrophobic |
| C16 | CG | LYS- 896 | 3.59 | 0 | Hydrophobic |
| C17 | CD | LYS- 896 | 4.46 | 0 | Hydrophobic |
| C12 | CE2 | TYR- 899 | 4.05 | 0 | Hydrophobic |
| C16 | CD2 | TYR- 899 | 3.65 | 0 | Hydrophobic |
| C18 | CG2 | ILE- 940 | 4 | 0 | Hydrophobic |