



1.000 Å
X-ray
2009-03-04
| Name: | Aldose reductase |
|---|---|
| ID: | ALDR_HUMAN |
| AC: | P15121 |
| Organism: | Homo sapiens |
| Reign: | Eukaryota |
| TaxID: | 9606 |
| EC Number: | 1.1.1.21 |
| Chain Name: | Percentage of Residues within binding site |
|---|---|
| A | 100 % |
| B-Factor: | 6.583 |
|---|---|
| Number of residues: | 32 |
| Including | |
| Standard Amino Acids: | 30 |
| Non Standard Amino Acids: | 1 |
| Water Molecules: | 1 |
| Cofactors: | NDP |
| Metals: | |
| Ligandability | Volume (Å3) |
|---|---|
| 1.029 | 391.500 |
| % Hydrophobic | % Polar |
|---|---|
| 62.07 | 37.93 |
| According to VolSite | |
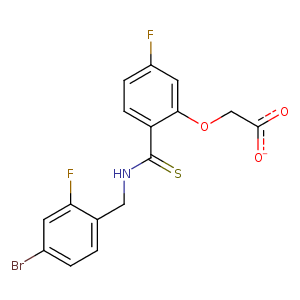
| HET Code: | LDT |
|---|---|
| Formula: | C16H11BrF2NO3S |
| Molecular weight: | 415.229 g/mol |
| DrugBank ID: | DB08084 |
| Buried Surface Area: | 75.24 % |
| Polar Surface area: | 93.48 Å2 |
| Number of | |
|---|---|
| H-Bond Acceptors: | 4 |
| H-Bond Donors: | 1 |
| Rings: | 2 |
| Aromatic rings: | 2 |
| Anionic atoms: | 1 |
| Cationic atoms: | 0 |
| Rule of Five Violation: | 0 |
| Rotatable Bonds: | 7 |
| X | Y | Z |
|---|---|---|
| 16.5343 | -7.21663 | 15.082 |
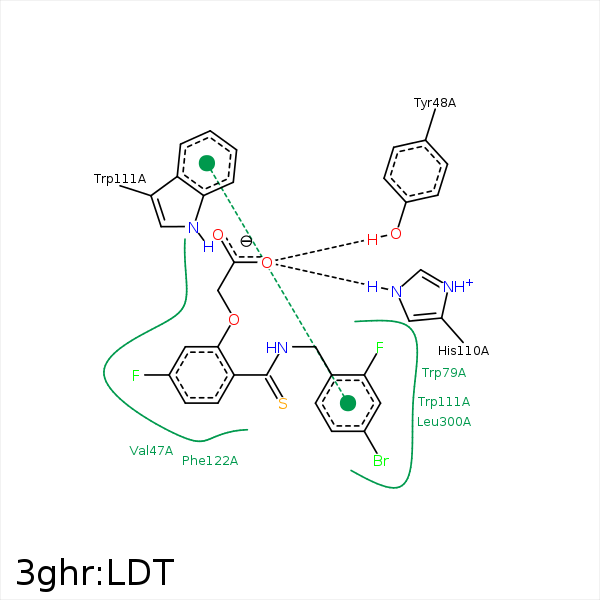
 Image generated by PoseView
Image generated by PoseView



Represent the protein/ligand binding mode, centered on the ligand
Dashed lines represents hydrogen bonds and metal interactions
Green residue labels for amino acids with hydrophobic contacts (green lines) to the ligand

| Ligand | Protein | Interaction | |||
|---|---|---|---|---|---|
| Atom | Atom | Residue | Distance (Å) | Angle (°) | Type |
| C20 | CD2 | TRP- 20 | 3.53 | 0 | Hydrophobic |
| C6 | CG2 | VAL- 47 | 4.08 | 0 | Hydrophobic |
| F9 | CG1 | VAL- 47 | 3.63 | 0 | Hydrophobic |
| F9 | CD1 | TYR- 48 | 3.6 | 0 | Hydrophobic |
| C20 | CE1 | TYR- 48 | 4.14 | 0 | Hydrophobic |
| O33 | OH | TYR- 48 | 2.76 | 159.6 | H-Bond (Protein Donor) |
| O33 | NE2 | HIS- 110 | 2.68 | 154.23 | H-Bond (Protein Donor) |
| BR8 | CE3 | TRP- 111 | 3.84 | 0 | Hydrophobic |
| C13 | CZ2 | TRP- 111 | 3.67 | 0 | Hydrophobic |
| F14 | CH2 | TRP- 111 | 3.24 | 0 | Hydrophobic |
| C25 | CB | TRP- 111 | 4.49 | 0 | Hydrophobic |
| O34 | NE1 | TRP- 111 | 3.06 | 155.04 | H-Bond (Protein Donor) |
| DuAr | DuAr | TRP- 111 | 3.45 | 0 | Aromatic Face/Face |
| BR8 | CG2 | THR- 113 | 3.97 | 0 | Hydrophobic |
| BR8 | CZ | PHE- 115 | 3.97 | 0 | Hydrophobic |
| C13 | CB | CYS- 298 | 4.48 | 0 | Hydrophobic |
| F14 | CB | CYS- 298 | 4.41 | 0 | Hydrophobic |
| C20 | SG | CYS- 298 | 4.27 | 0 | Hydrophobic |
| C24 | CB | LEU- 300 | 3.99 | 0 | Hydrophobic |
| C26 | CD2 | LEU- 300 | 4.11 | 0 | Hydrophobic |
| F14 | CB | LEU- 300 | 3.55 | 0 | Hydrophobic |
| BR8 | CB | CYS- 303 | 3.97 | 0 | Hydrophobic |
| C25 | SG | CYS- 303 | 4.24 | 0 | Hydrophobic |
| BR8 | CD1 | TYR- 309 | 4.46 | 0 | Hydrophobic |
| C20 | C4N | NDP- 318 | 3.61 | 0 | Hydrophobic |