



2.050 Å
X-ray
2015-06-13
| Name: | UDP-N-acetylglucosamine--peptide N-acetylglucosaminyltransferase 110 kDa subunit |
|---|---|
| ID: | OGT1_HUMAN |
| AC: | O15294 |
| Organism: | Homo sapiens |
| Reign: | Eukaryota |
| TaxID: | 9606 |
| EC Number: | / |
| Chain Name: | Percentage of Residues within binding site |
|---|---|
| A | 100 % |
| C | 0 % |
| B-Factor: | 32.819 |
|---|---|
| Number of residues: | 47 |
| Including | |
| Standard Amino Acids: | 45 |
| Non Standard Amino Acids: | 0 |
| Water Molecules: | 2 |
| Cofactors: | |
| Metals: | |
| Ligandability | Volume (Å3) |
|---|---|
| 0.492 | 405.000 |
| % Hydrophobic | % Polar |
|---|---|
| 35.83 | 64.17 |
| According to VolSite | |
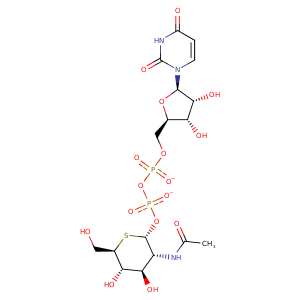
| HET Code: | 12V |
|---|---|
| Formula: | C17H25N3O16P2S |
| Molecular weight: | 621.403 g/mol |
| DrugBank ID: | - |
| Buried Surface Area: | 70.93 % |
| Polar Surface area: | 341.76 Å2 |
| Number of | |
|---|---|
| H-Bond Acceptors: | 17 |
| H-Bond Donors: | 7 |
| Rings: | 3 |
| Aromatic rings: | 0 |
| Anionic atoms: | 2 |
| Cationic atoms: | 0 |
| Rule of Five Violation: | 3 |
| Rotatable Bonds: | 10 |
| X | Y | Z |
|---|---|---|
| 12.4707 | -45.8646 | -29.3344 |

 Image generated by PoseView
Image generated by PoseView



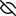
Represent the protein/ligand binding mode, centered on the ligand
Dashed lines represents hydrogen bonds and metal interactions
Green residue labels for amino acids with hydrophobic contacts (green lines) to the ligand

| Ligand | Protein | Interaction | |||
|---|---|---|---|---|---|
| Atom | Atom | Residue | Distance (Å) | Angle (°) | Type |
| O7' | NE2 | HIS- 498 | 3.04 | 158.42 | H-Bond (Protein Donor) |
| C3B | CG | PRO- 559 | 4.35 | 0 | Hydrophobic |
| S5' | CB | PRO- 559 | 3.37 | 0 | Hydrophobic |
| C5' | CD1 | LEU- 563 | 4.17 | 0 | Hydrophobic |
| O4' | O | LEU- 653 | 2.58 | 166.99 | H-Bond (Ligand Donor) |
| C8' | CG | PRO- 656 | 4.1 | 0 | Hydrophobic |
| C4' | CZ | PHE- 694 | 4.42 | 0 | Hydrophobic |
| O2A | NE2 | GLN- 839 | 2.76 | 160.57 | H-Bond (Protein Donor) |
| O1B | NZ | LYS- 842 | 3.28 | 0 | Ionic (Protein Cationic) |
| O2B | NZ | LYS- 842 | 2.69 | 0 | Ionic (Protein Cationic) |
| O2B | NZ | LYS- 842 | 2.69 | 171.79 | H-Bond (Protein Donor) |
| N3 | O | ALA- 896 | 2.76 | 162.67 | H-Bond (Ligand Donor) |
| O4 | N | ALA- 896 | 2.83 | 175.11 | H-Bond (Protein Donor) |
| C1B | CD | LYS- 898 | 4.46 | 0 | Hydrophobic |
| O2' | NZ | LYS- 898 | 2.75 | 159.58 | H-Bond (Protein Donor) |
| O3B | NZ | LYS- 898 | 3 | 121.76 | H-Bond (Protein Donor) |
| C8' | SG | CYS- 917 | 3.81 | 0 | Hydrophobic |
| C1' | CB | HIS- 920 | 4.41 | 0 | Hydrophobic |
| C3' | CB | HIS- 920 | 3.54 | 0 | Hydrophobic |
| O1B | N | HIS- 920 | 2.9 | 154.5 | H-Bond (Protein Donor) |
| O3' | ND1 | HIS- 920 | 2.63 | 158.09 | H-Bond (Ligand Donor) |
| O1' | OG1 | THR- 921 | 2.77 | 155.85 | H-Bond (Protein Donor) |
| O1' | N | THR- 921 | 2.97 | 145.75 | H-Bond (Protein Donor) |
| C1' | CB | THR- 921 | 4.36 | 0 | Hydrophobic |
| C3B | CG2 | THR- 921 | 3.84 | 0 | Hydrophobic |
| O1B | N | THR- 922 | 2.94 | 170.14 | H-Bond (Protein Donor) |
| O1B | OG1 | THR- 922 | 2.73 | 160.89 | H-Bond (Protein Donor) |
| O3A | OG1 | THR- 922 | 3.45 | 129.94 | H-Bond (Protein Donor) |
| O2' | OD2 | ASP- 925 | 2.61 | 168.57 | H-Bond (Ligand Donor) |