



2.300 Å
X-ray
2003-03-28
| Name: | Deoxyribodipyrimidine photo-lyase |
|---|---|
| ID: | PHR_SYNP6 |
| AC: | P05327 |
| Organism: | Synechococcus sp. |
| Reign: | Bacteria |
| TaxID: | 269084 |
| EC Number: | 4.1.99.3 |
| Chain Name: | Percentage of Residues within binding site |
|---|---|
| A | 100 % |
| B-Factor: | 23.530 |
|---|---|
| Number of residues: | 47 |
| Including | |
| Standard Amino Acids: | 46 |
| Non Standard Amino Acids: | 0 |
| Water Molecules: | 1 |
| Cofactors: | |
| Metals: | |
| Ligandability | Volume (Å3) |
|---|---|
| 1.139 | 1221.750 |
| % Hydrophobic | % Polar |
|---|---|
| 41.16 | 58.84 |
| According to VolSite | |
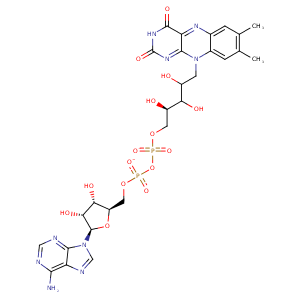
| HET Code: | FAD |
|---|---|
| Formula: | C27H31N9O15P2 |
| Molecular weight: | 783.534 g/mol |
| DrugBank ID: | DB03147 |
| Buried Surface Area: | 75.79 % |
| Polar Surface area: | 381.7 Å2 |
| Number of | |
|---|---|
| H-Bond Acceptors: | 22 |
| H-Bond Donors: | 7 |
| Rings: | 6 |
| Aromatic rings: | 3 |
| Anionic atoms: | 2 |
| Cationic atoms: | 0 |
| Rule of Five Violation: | 3 |
| Rotatable Bonds: | 13 |
| X | Y | Z |
|---|---|---|
| 48.2528 | 23.1122 | 53.8134 |
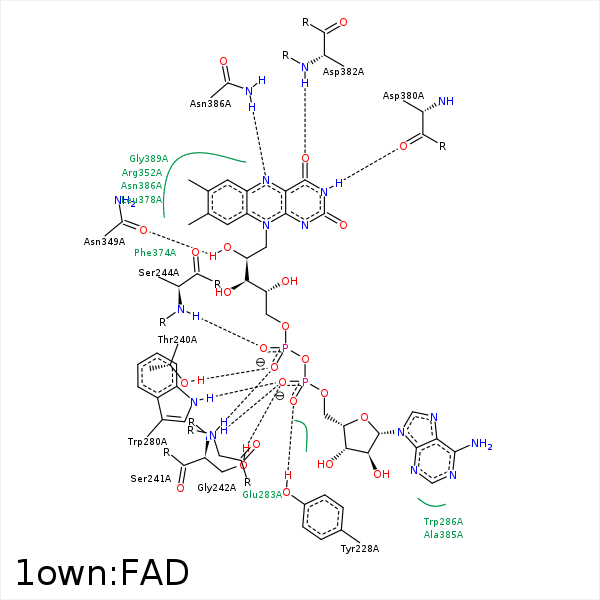
 Image generated by PoseView
Image generated by PoseView



Represent the protein/ligand binding mode, centered on the ligand
Dashed lines represents hydrogen bonds and metal interactions
Green residue labels for amino acids with hydrophobic contacts (green lines) to the ligand

| Ligand | Protein | Interaction | |||
|---|---|---|---|---|---|
| Atom | Atom | Residue | Distance (Å) | Angle (°) | Type |
| O2A | OH | TYR- 228 | 2.59 | 134.1 | H-Bond (Protein Donor) |
| O2P | OG1 | THR- 240 | 2.71 | 141.16 | H-Bond (Protein Donor) |
| O1A | OG | SER- 241 | 2.82 | 152.7 | H-Bond (Protein Donor) |
| O1A | N | SER- 241 | 2.93 | 161.5 | H-Bond (Protein Donor) |
| O2P | N | GLY- 242 | 2.67 | 150.79 | H-Bond (Protein Donor) |
| C3B | CB | SER- 244 | 4.42 | 0 | Hydrophobic |
| C5' | CB | SER- 244 | 3.51 | 0 | Hydrophobic |
| O1P | N | SER- 244 | 2.92 | 133.79 | H-Bond (Protein Donor) |
| C4B | CD1 | LEU- 247 | 3.8 | 0 | Hydrophobic |
| O1A | NE1 | TRP- 280 | 2.97 | 168.81 | H-Bond (Protein Donor) |
| C5B | CZ2 | TRP- 280 | 3.71 | 0 | Hydrophobic |
| C5B | CB | GLU- 283 | 4.46 | 0 | Hydrophobic |
| C4B | CD2 | LEU- 284 | 4 | 0 | Hydrophobic |
| C2B | CD | ARG- 287 | 4.43 | 0 | Hydrophobic |
| C1B | CB | ARG- 287 | 4.19 | 0 | Hydrophobic |
| C5' | CZ2 | TRP- 346 | 4.37 | 0 | Hydrophobic |
| C2' | CB | ASN- 349 | 4.02 | 0 | Hydrophobic |
| C4' | CB | ASN- 349 | 4.31 | 0 | Hydrophobic |
| O2' | OD1 | ASN- 349 | 2.66 | 155.56 | H-Bond (Ligand Donor) |
| N5 | NH1 | ARG- 352 | 3.36 | 140.06 | H-Bond (Protein Donor) |
| C6 | CD | ARG- 352 | 4.24 | 0 | Hydrophobic |
| C8M | CB | ARG- 352 | 3.79 | 0 | Hydrophobic |
| C2' | CD | ARG- 352 | 4.43 | 0 | Hydrophobic |
| C9A | CD | ARG- 352 | 3.83 | 0 | Hydrophobic |
| C8 | CB | ARG- 352 | 3.59 | 0 | Hydrophobic |
| C8M | CG | MET- 353 | 3.79 | 0 | Hydrophobic |
| C7M | CB | ALA- 356 | 3.72 | 0 | Hydrophobic |
| C7M | CE2 | PHE- 374 | 3.89 | 0 | Hydrophobic |
| N3 | O | ASP- 380 | 2.89 | 154.32 | H-Bond (Ligand Donor) |
| O4 | N | ASP- 382 | 2.88 | 162.98 | H-Bond (Protein Donor) |
| N5 | ND2 | ASN- 386 | 3.17 | 175.09 | H-Bond (Protein Donor) |
| C7M | CE2 | TRP- 390 | 3.83 | 0 | Hydrophobic |