



2.300 Å
X-ray
2001-11-06
| Name: | NAD(P)H dehydrogenase [quinone] 1 |
|---|---|
| ID: | NQO1_HUMAN |
| AC: | P15559 |
| Organism: | Homo sapiens |
| Reign: | Eukaryota |
| TaxID: | 9606 |
| EC Number: | 1.6.5.2 |
| Chain Name: | Percentage of Residues within binding site |
|---|---|
| B | 48 % |
| D | 52 % |
| B-Factor: | 32.540 |
|---|---|
| Number of residues: | 29 |
| Including | |
| Standard Amino Acids: | 28 |
| Non Standard Amino Acids: | 1 |
| Water Molecules: | 0 |
| Cofactors: | FAD |
| Metals: | |
| Ligandability | Volume (Å3) |
|---|---|
| 1.265 | 813.375 |
| % Hydrophobic | % Polar |
|---|---|
| 45.64 | 54.36 |
| According to VolSite | |
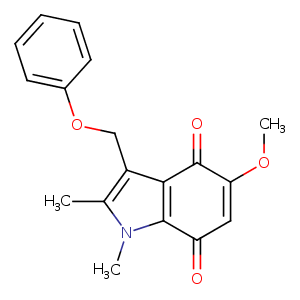
| HET Code: | 340 |
|---|---|
| Formula: | C18H17NO4 |
| Molecular weight: | 311.332 g/mol |
| DrugBank ID: | DB03626 |
| Buried Surface Area: | 73.85 % |
| Polar Surface area: | 57.53 Å2 |
| Number of | |
|---|---|
| H-Bond Acceptors: | 4 |
| H-Bond Donors: | 0 |
| Rings: | 3 |
| Aromatic rings: | 2 |
| Anionic atoms: | 0 |
| Cationic atoms: | 0 |
| Rule of Five Violation: | 0 |
| Rotatable Bonds: | 4 |
| X | Y | Z |
|---|---|---|
| -28.6041 | -32.3273 | -27.387 |
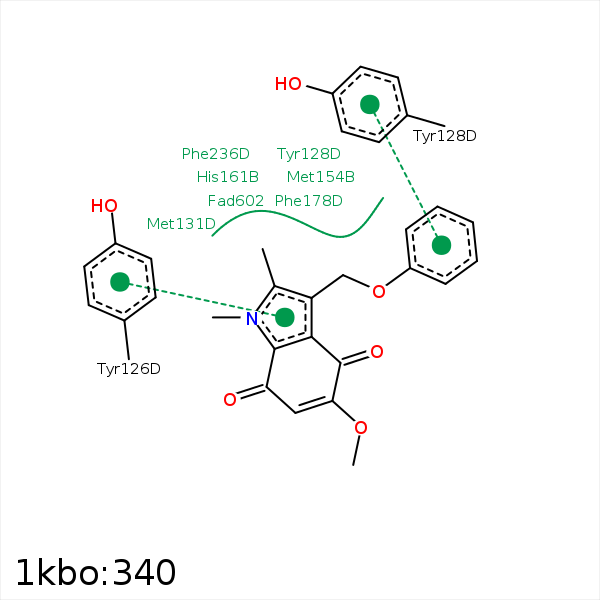
 Image generated by PoseView
Image generated by PoseView



Represent the protein/ligand binding mode, centered on the ligand
Dashed lines represents hydrogen bonds and metal interactions
Green residue labels for amino acids with hydrophobic contacts (green lines) to the ligand

| Ligand | Protein | Interaction | |||
|---|---|---|---|---|---|
| Atom | Atom | Residue | Distance (Å) | Angle (°) | Type |
| C45 | CG | PRO- 68 | 3.48 | 0 | Hydrophobic |
| C12 | CZ2 | TRP- 105 | 3.38 | 0 | Hydrophobic |
| C37 | CZ3 | TRP- 105 | 4.23 | 0 | Hydrophobic |
| C37 | CE1 | PHE- 106 | 3.49 | 0 | Hydrophobic |
| C12 | CZ | TYR- 126 | 3.87 | 0 | Hydrophobic |
| DuAr | DuAr | TYR- 128 | 3.99 | 0 | Aromatic Face/Face |
| C26 | CB | TYR- 128 | 3.95 | 0 | Hydrophobic |
| C26 | SD | MET- 131 | 3.45 | 0 | Hydrophobic |
| C25 | CE | MET- 154 | 3.58 | 0 | Hydrophobic |
| C19 | CZ | PHE- 178 | 4.47 | 0 | Hydrophobic |
| C37 | CZ | PHE- 178 | 3.46 | 0 | Hydrophobic |
| C27 | CZ | PHE- 236 | 3.5 | 0 | Hydrophobic |
| C12 | C6 | FAD- 602 | 4.23 | 0 | Hydrophobic |
| C45 | C1' | FAD- 602 | 4.17 | 0 | Hydrophobic |